
Direct Competitive cAMP ELISA Kit Based on Monoclonal Anti-cAMP Antibody
Introduction
Adenosine 3', 5'-cyclic monophosphate (cAMP) is a key second messenger molecule in intracellular signal transduction. Monitoring cAMP levels is one of the most common ways to screen for agonists and antagonists of G protein–coupled receptors(GPCRs). Bioyeargene cAMP ELISA Kit is based on the competition between HRP-labeled cAMP and free cAMP for fixed amounts of mouse monoclonal anti-cAMP antibody(cAMP MAb) binding sites. Because the concentration of the HRP-labeled cAMP is held constant while the concentration of cAMP varies, the amount of HRP-labeled cAMP that is able to bind to the cAMP MAb will be inversely proportional to the concentration of cAMP in the well. This antigen-antibody complex binds to the goat anti-mouse IgG that has been previously attached to the well. The plate is washed to remove any unbound reagents and then developed. The ELISA typically displays an IC50(50% B/B0) of approximately 12 pmol/mL and a detection limit of approximately 0.15 pmol/mL.
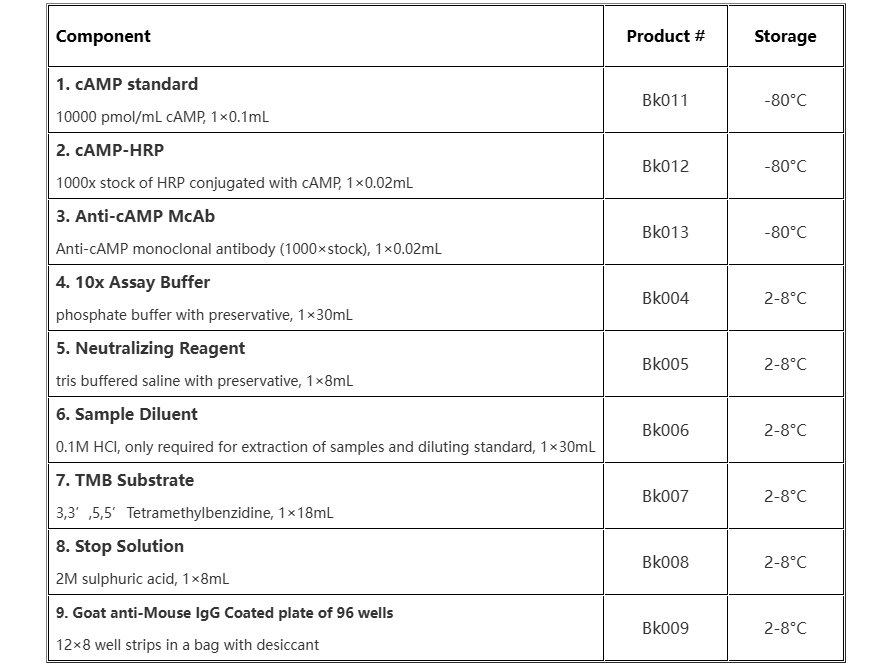
Reagents & Materials Provided
Storage & Stability
Store kit at 4°C immediately upon receipt, apart from Standard、HRP conjugate、antibody, which should be stored at -80°C.
Materials & Equipment Required But Not Provided
Standard microplate reader - capable of reading at 450 nm, preferably with correction at 630 nm
Adjustable pipettes and pipette tips. Multichannel pipettes are recommended when large sample sets are being analyzed
Eppendorf tubes
Microtiter plate rotator
Absorbent paper for blotting
Deionized water
Sample Handling
Plasma, serum, whole blood, tissue homogenates and other samples often contain phosphodiesterases and large amount of proteins (e.g.globulins, albumin)which may interfere with the assay. However, preparing samples in 0.1M HCl can generally inactivate phosphodiesterases and lower the concentration of proteins, making the samples suitable for the assay. Both phosphodiesterases and proteins can also be removed by 5% TCA precipitation or by using 10 kDa molecular weight cut off microcentrifuge filters.
Tissue samples: It is important to rapidly freeze tissues using liquid nitrogen after collection of tissue samples. Then weigh the frozen tissue and add 5-10 volume of 0.1M HCl. Homogenize the sample on ice using a homogenizer. Centrifuge at 3000rpm at room temperature for 5min. The supernatant may be assayed directly and can then be diluted in the 0.1M HCl.
Cells samples: After removing the culture medium, cells can be collected and treated with 0.1M HCl. Incubate at room temperature for 20 minutes and ensure complete cell lysis. Centrifuge at 3000 rpm at room temperature for 5 min, then the supernatants may be used directly in the assay.
Urine, Serum and Culture Medium Samples: After treating 1mL of the supernatant media with 10 µL of concentrated hydrochloric acid, Centrifuge Urine and plasma for 5 minutes at 3000 rpm at room temperature. The supernatants can then be used directly in the assay or diluted to a suitable multiple.
Procedural Notes
- 1.Bring all kit reagents to warm to room temperature(26±2℃) before use.
- 2.Pipet standards and samples to the bottom of the wells.
- 3.Add the reagents to the side of the well to avoid contamination.
- 4.This kit uses break-apart microtiter strips, which allow the user to measure as many samples as desired. Unused wells must be kept desiccated at 4°C in the sealed bag provided. The wells should be used in the frame provided.
- 5.Prior to addition of substrate, ensure that there is no residual wash buffer in the wells.
- 6.Stop solution is caustic; care should be taken in use.
Reagent Preparation
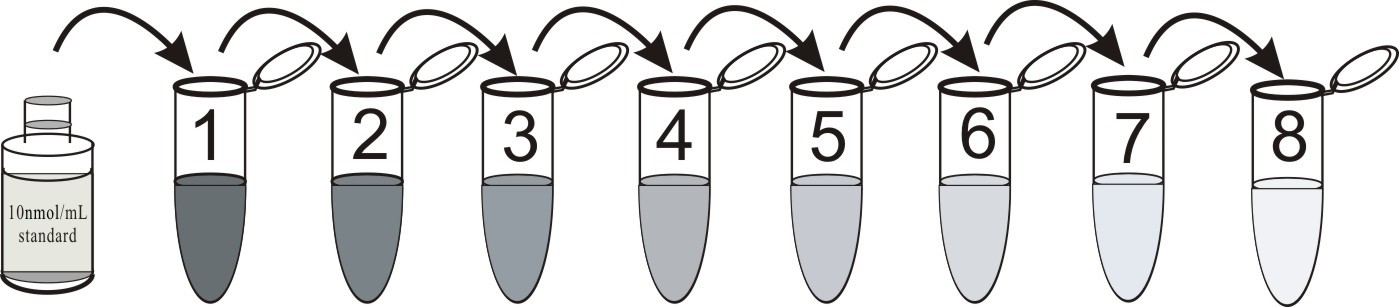
1. cAMP standard solution
Label eight tubes 1# through 8#. Pipet 980 μL 0.1M HCl into tube 1# and 500 μL 0.1M HCl into tubes 2-8#. Add 20 μL of the 10000 pmol/mL standard to tube 1#. Vortex thoroughly. Add 500 μL of tube 1# to tube 2# and vortex thoroughly. Continue this for tubes 3# through 8#.
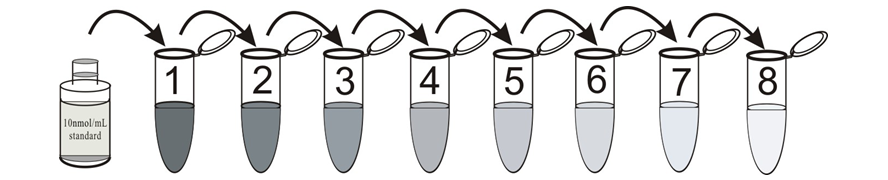
The concentration of cAMP in tube 1# through 9# will be 200, 100, 50, 25, 12.5, 6.25, 3.125, 1.56 pmol/mL respectively. Diluted standards should be used within 15 minutes of preparation. Label one tube as the Zero Standard(0 pmol/mL, maximum binding/B0). Pipet 500 μL 0.1M HCl into this tube.
2. Assay buffer
prepare the wash buffer by diluting 30 mL of the supplied concentrate with 270 mL of deionized water. This can be stored at room temperature until the kit expiration date, or for 3 months, whichever is earlier.
3. cAMP-HRP Conjugate
Make 1:1000 dilution with assay buffer to have 1× cAMP-HRP conjugate working solution before use.
4. Conjugate 1:20 Dilution for Total Activity Measurement
Prepare the Conjugate 1:20 Dilution by diluting 10 μL of the Conjugate working solution with 190 μL of the assay buffer. This 1:20 dilution is intended for use in the Total Activity wells only.
5. Anti-cAMP McAb
Make 1:1000 dilution with assay buffer to have 1× Anti-cAMP McAb conjugate working solution before use.
Assay Procedure
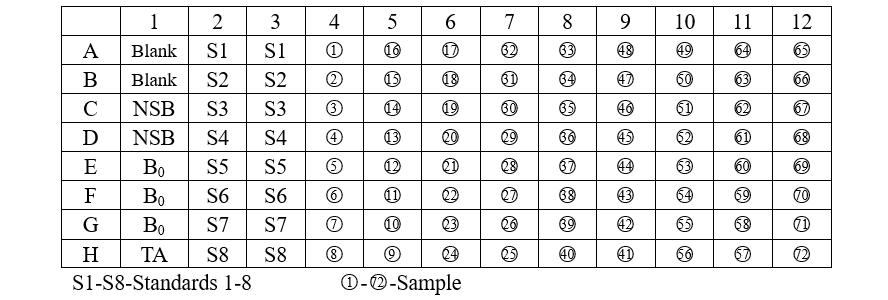
Add various reagents to the wells according to the following table:

1. Add 50 µL of the Neutralizing Buffer to each well, except the TA (Total Activity) and Blank wells.
2. Pipet 50 µL of 0.1M HCl into the NSB (Non-Specific Binding) and the maximum binding(B0) wells.
3. Pipet 50 µL of Standards and samples to the appropriate wells.
4. Pipet 50 µL of assay buffer into the NSB wells.
5. Pipet 50 µL of Conjugate into each well except the TA and Blank wells.
6. Pipet 50 µL of Antibody into each well, except the Blank, TA and NSB wells.
7. Incubate the plate at room temperature(26 ± 2℃) for 2 hours.
8. Wash 4 times with 260 µL Assay Buffer each well. Completely empty the wells by tapping the plate on a new paper towel.
9. Add 5 µL of the Conjugate to the TA wells.
10. Add 150 µL of the Substrate solution to each well. Incubate at room temperature for about 10 minutes without shaking.
11. Stop the reaction by adding 50 µL of Stop Solution to each well and read at 450 nm.
Calculation & Standard Curve
Calculate the average Net Optical Density (OD) bound for each standard and sample by subtracting the average NSB OD from the average OD bound:
Average Net OD = Average Bound OD - Average NSB OD
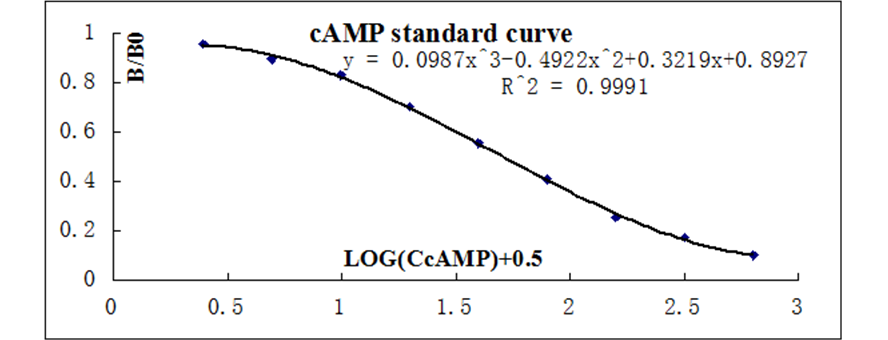
The data should be plotted as B/B0 versus log concentration using curve fitting routines such as exponential logistic curve fitting or three order polynomial linear regression curve-fit.
Calculate the B/B0 value for each sample. Determine the concentration of each sample using the equation obtained from the standard curve plot.
The lower limit of detection was determined as the concentration corresponding to a signal two standard deviations above the mean of the zero standard. The LOD is ~0.15 pmol/mL.